At a time of challenges that call for change, it is necessary to place health at the heart of public policy.[1] The importance of pharmaceutical innovation cannot be underestimated, in the interests of public health and the economy alike. By responding to medical needs that are poorly covered or not at all,[2] it offers therapeutic solutions for many pathologies that were previously untreated.[3]These multiple therapeutic advances are the fruit of ongoing efforts by the global pharmaceutical industry, which has played a decisive role in promoting innovation over the past ten years.[4]
*LEEM – Les Entreprises du Médicament (www.leem.org)
Medicines account for 15% of healthcare expenditure, making the pharmaceutical industry a strategic sector for developed countries,3 but one that nevertheless faces a number of challenges.
As patents fall into the public domain, pharmaceutical companies are being forced to step up their medical research efforts or withdraw from certain less promising sectors. Between drug reimbursement schemes, social security deficits and reduced production costs, the industry has to adapt to new production methods.
Policies to support innovation are therefore essential to accompany and encourage the development of the sector. So, what are these innovation support policies, and what impact do they have on the pharmaceutical industry?
What policies are in place to support pharmaceutical innovation?
An innovation support policy is a set of measures and initiatives put in place by governments or organizations to encourage, facilitate and create a favorable environment for innovation.
Horizon Europe is the European Union’s framework program for the specific funding of research and innovation, covering the period 2021-2027. It aims to strengthen the Union’s scientific and technological foundations, give concrete expression to its strategic political priorities, and respond to global issues.[5] Among the pillars of Horizon Europe, two of them support pharmaceutical innovation:[6]
- Pillar I supports basic research projects and infrastructure development, through initiatives such as the European Research Council (ERC) and Marie Skłodowska-Curie Actions.
- Pillar III [PR3] develops innovation in Europe and promotes the integration of players, through the European Innovation Council (EIC) and the European Institute of Innovation and Technology (EIT).
In addition to Horizon Europe, there are other European initiatives encompassing a variety of programs and partnerships. These include: Incubators:

- Since 2012, the program has been supporting innovative techniques, both costly and inexpensive. It demonstrates their viability and usefulness, establishes their place and impact, and facilitates the evaluation and development of innovation.2
- The EU’s pharmaceutical policy, reformed in 2023, promotes and rewards innovation while strengthening pharmaceutical sovereignty, in particular by securing the supply of medicines.[7]
- Subsidies and tax incentives: schemes such as the research tax credit and innovation tax credit foster private investment in R&D and innovation. These financial advantages encourage growth and are monitored by the Organization for Economic Cooperation and Development (OECD).[8]
Public health policies also employ other means via drug regulatory bodies.[9] Here are a few examples:
- Marketing authorization (MA): verifies a drug’s conformity and defines its use.
- The Transparency Commission: evaluates the usefulness of a drug and determines its reimbursement rate.
- For special cases:Conditional MA by the European Medicines Agency (EMA) and the European Commission: This fast-track procedure is used in emergencies (such as COVID-19) and allows developers to submit additional data after initial authorization.[10]
- Early Access Authorization (Autorisation d’Accès Précoce, AAP) by the Agence Nationale de Sécurité du Médicament (ANSM) and the Haute Autorité de Santé (HAS) since July 2021: This French system enables patients with[PR7] no therapeutic alternative to gain early access to innovative medicines prior to marketing authorization. Between 2021 and 2024, 78% of decisions were positive (122 AAPs out of 172), mainly in oncology-hematology, allowing access to these treatments for over 120,000 patients in therapeutic deadlock situations.[11]
Policy impact on pharmaceutical innovation: challenges and opportunities
There are many measures in place to support and enhance development of pharmaceutical innovation, but several challenges remain.
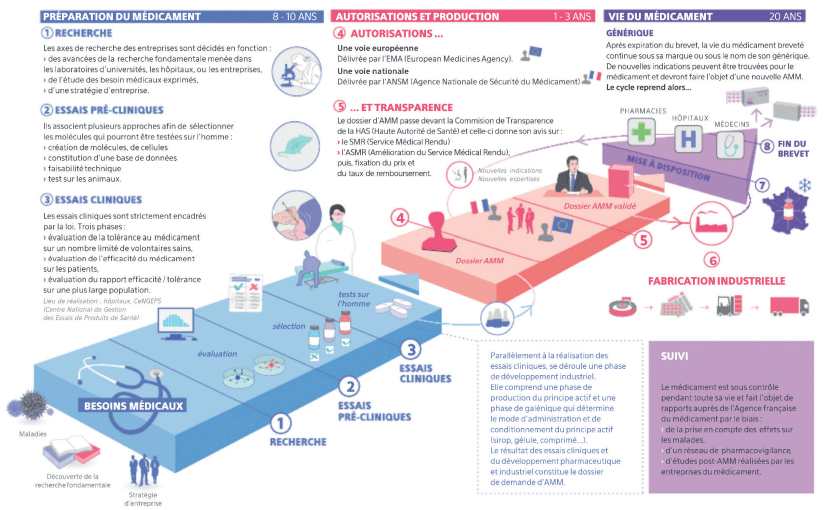
- The innovation process in the biopharmaceutical sector is a long one. It can take up to 10-12 years from initial validation through to market launch. This makes it difficult to predict timescales and costs.4
- Although medicines are approved within the European Union, they do not always reach patients as quickly as they should. Access to these medicines varies considerably from one member state to another, creating healthcare inequalities.10
- The response to unmet medical needs is fragile, prices of innovative treatments are high, and drug shortages put patients and healthcare systems at risk.10
- New rules now require consideration of the environmental impact of drug production, making regulation even more complex.10
- Existing policies should be strengthened by increasing funding and simplifying administrative procedures. [PR9]
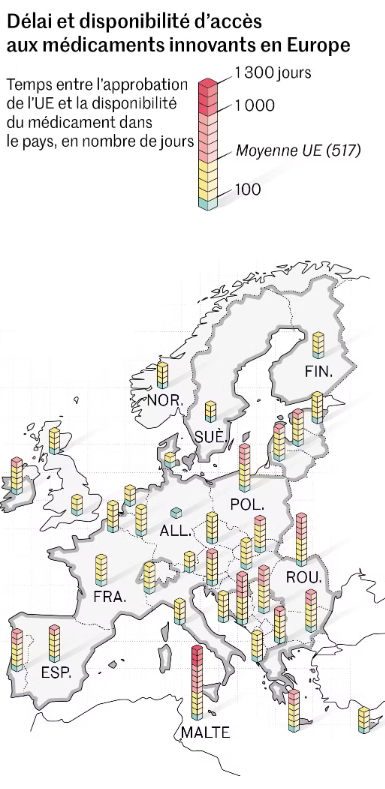
*Time to access and availability of innovative medicines in Europe Le Monde, “These innovative medicines that are not available in France to treat patients”, June 4, 2023.
Opportunities exist to overcome these challenges:
- Establishing more partnerships is essential to support the early stages of research, as public investment alone is not enough to ensure the development of a sustainable innovative sector.4
- Promoting international collaboration.
- Transparency in the pharmaceutical chain is crucial to the production of medicines in Europe. It enables a better understanding of the issues at stake, restores balance in negotiations between regulators, and improves analysis of the measures to be adopted.1
Pharmaceutical innovation represents a major opportunity to transform the healthcare delivery system profoundly.3
Policies to support innovation have a positive impact on pharmaceutical production and public health. They stimulate research and development of new medicines, particularly in the field of excipients. For each new molecule, the best combination of excipients must be found to ensure the drug’s efficacy [PR12] . Policies therefore play a crucial role by supporting the industry in this process and creating a favorable environment.
Challenges remain, however, particularly in international cooperation and universal access to healthcare. More than ever before, the geopolitical context in the U.S. is putting a strain on medical research. The need for funding to alleviate these problems is growing, and some production plants may have to be relocated. Collaboration between the various players in the sector is therefore essential to overcome these obstacles.
[1] OTMeds, Une politique pragmatique au service du droit à la santé, 2022
[2] Ministère de la Santé, Programme de soutien aux techniques innovantes, coûteuses ou non (PSTIC), 2025
[3] Haut Conseil de la Santé Publique (HCSP), L’industrie pharmaceutique : propositions pour accompagner l’innovation, 2016
[4] Tim Wilsdon et Eva Fiz, Encouraging pharmaceutical innovation in middle-income countries, 2013
[5] Ministère de l’enseignement supérieur et de la Recherche, Horizon Europe, c’est quoi ?, 2020
[6] Ministère de l’enseignement supérieur et de la Recherche, Autres financements en santé, 2022
[7] Parlement Européen, Politique pharmaceutique de l’UE: les députés soutiennent une réforme globale, 2024
[8] OCDE, Incitations fiscales à la R&D, 2024
[9] ADSP, Médicament et santé publique, 2000
[10] Commission Européenne, Union européenne de la santé: La Commission propose une réforme des produits pharmaceutiques pour des médicaments plus accessibles, plus abordables et plus innovants, 2023
[11] HAS, Accès précoce des médicaments : un bilan positif après deux ans de mise en place du dispositif, 2024